Water accumulation can cause greater problems. Gasoline can only contain about 0.02 % water by volume before the water will separate. This memo from the Environmental Protection Agency details some of the issues (thanks to Robert Rapier who tracked this down for me). If a car is left outside where it can go through many hot/cool cycles condensation can increase the amount of water in the tank. When it gets cold in the winter time the water can freeze or simply stall the engine as it drops to the bottom of the tank. Water expands when it freezes so you don't want freezing to occur in your fuel lines or pump.
 Ethanol mixed with gasoline complicates the situation. Ethanol likes to absorb water. Anyone who knows anything about liquor probably is aware that ethanol and water are miscible, i.e. they will mix in any proportion. For a vehicle running on pure ethanol this wouldn't be a serious problem. The presence of water in the fuel reduces fuel economy because it is dead weight that needs to be vapourized and brought up to high temperature in the cylinder. However, that's all it does.
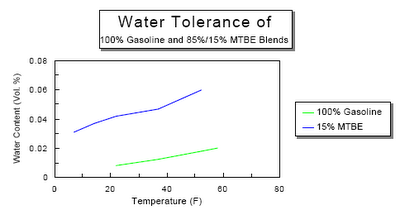
Ethanol mixed with gasoline complicates the situation. Ethanol likes to absorb water. Anyone who knows anything about liquor probably is aware that ethanol and water are miscible, i.e. they will mix in any proportion. For a vehicle running on pure ethanol this wouldn't be a serious problem. The presence of water in the fuel reduces fuel economy because it is dead weight that needs to be vapourized and brought up to high temperature in the cylinder. However, that's all it does. When you mix gasoline, ethanol, and water you get a ternary solution as long as the water content is low enough. As the proportion of water increases it will reach a critical point at which time the ethanol and water will separate from the gasoline and form a new solution. A water/ethanol solution is heavier than gasoline or water, so it will drop to the bottom of the tank.
When you mix gasoline, ethanol, and water you get a ternary solution as long as the water content is low enough. As the proportion of water increases it will reach a critical point at which time the ethanol and water will separate from the gasoline and form a new solution. A water/ethanol solution is heavier than gasoline or water, so it will drop to the bottom of the tank. As you can see from the graph the tolerance drops with lower temperatures. If water manages build up in the tank over the summer and fall then in the winter as the temperature drops so does the tolerance. Where previously you were just sipping gasohol with 0.5 % water content you might end up with half a litre of ethanol and water in the bottom of your tank after an overnight cold snap. Then when the driver stomps on the pedal to get the power of the engine along with the electric drive the engine will turn over but then splutter and not fire.
It's generally quite difficult to separate water and ethanol. Ethanol has a higher vapour pressure than water (which means it likes to evaporate faster) and it's more polar. It's not practical to use any sort of desiccant to remove water from gasohol. The best solution is generally to keep the tank full and use a better seal for the gas cap. Stations will also have to take steps to insure that their storage facilities don't allow water to infiltrate before they sell it to their customer. Overall it's a fairly minor problem that can be overcome with good design.
No comments:
Post a Comment