Radio-thermal generators (RTG) are a type of thermal battery that generate power by the decay of radioisotopes. RTGs powered by Plutonium-238 have been used on all the deep space probes, from Voyager to Cassini. These devices are much simpler than reactors, and can output about 600 W/kg for the many years needed to travel over interplanetary distances. While this is marginally sufficient for old space probes, it's not a sufficiently high power density to power ion drives or the like. The Soviets also used Sr-90 RTGs to power lighthouses (and have of course left them around the countryside).
While Plutonium-238 can be bred in a nuclear reactor fairly easily, I did start to wonder if there's a better radioisotope out there.
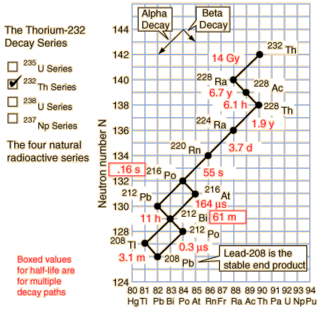
As you can see from the nice diagram I stole from Hyperphysics the natural Thorium-232 chain does contain a nice radioisotope decay chain starting at Radium-228. The overall half-life of the chain is only about 8.6 years and it drops 20 amu over that time.
So how much power does Ra-228 contain? Well I get to go through a radioisotope table and add up the decay energies. It works out to 38.575 MeV along the most popular decay path. If we convert that to moles it works out to an impressive 3.72 TJ/mol, or 16.3 TJ/kg! This is more energy than fissioning Uranium can produce, but less than light element fusion.
The power density is quite impressive too. While the radioisotope starts off slowly with the beta decay of Ra-228 to Ac-228, it quickly picks up speed after a couple years. This is a curious advantage for a space probe: you don't want the RTG to be producing power on the ground because radiating the heat is difficult.
If you approximate the decay as chain as Ra-228 → Th-228 (half-life 6.7 years) and then Th-228 → Pb-208 (half-life 1.9 years) then it's fairly straightforward to find the power output. The power output is in excess of 20 kW/kg from about 1.5 years to 10 years. This is a minor 3300 % improvement over the Plutonium radiothermal generators.
Since the nuclear reactions here are alpha and beta decay, only charged particles are emitted. These are relatively easy to capture in magnetic fields and produce electricity from directly, as opposed to the neutron production in fission reactors. You would of course want to capture the energy emitted with a very high efficiency because radiating it is difficult. Also, the half-life is quite short and the decay ends as relatively harmless lead, so there's not much of a waste problem.
There are some obvious drawbacks. For one, the power output is so massive that a block of Ra-228 wouldn't exist as a solid -- it would produce enough energy to vapourize itself. Thin films could probably exist as solids. It also has Radon-220 in its decay chain -- a very unnice gas. Then there's also the point that it's the ultimate material for a radiological weapon. But these are only minor pettifogging details next to the production question.
While Ra-228 occurs naturally from natural Thorium-232, notice the 14 billion year half-life of Thorium. Given the short half-life of the decay chain, the preponderance of Ra-228 in natural Thorium will be incredibly low. Unless someone has a way of inducing alpha decay I don't know about, this leaves one alternative: transmutation.
Transmutation generally involves smacking protons into a material to make a heavier one. Looking down the periodic table there isn't a suitable atom for Radium transmutation until we get to Bismuth-209. The other elements (Po, At, Rn, Fr) don't occur in any significant natural abundance. A suitable light atom counterpart would be Fluorine-19. Here we have one advantage at least: Bismuth-209 and Fluorine-19 are both the only naturally occurring isotopes of those two elements. As a plus, both are cheap. On the negative side, the result would be Rutherforium-228 which isn't a known isotope and it would quickly fly apart. I.e. we have too many protons, not enough neutrons. Boron-20 simply isn't realistic. A better idea might be to smash deuterium nucleai into Ra-226 (half-life 1600 years), which would produce Ac-228 (the third element of the Thorium decay chain). This would certainly ramp up the power output at the expense of duration.
So the objective is to accelerate a positively charged ion up to some relativistic velocity and smash it into a heavy atom target to make Ra-228. There's two basic particle accelerators: the cyclotron and the synchrotron. The biggest cyclotrons in the world, like TRIUMF, can accelerate protons up to 500 MeV/amu with very high beam densities.
The synchrotron can do better. The Brookhaven Relativistic Heavy Ion Collider (RHIC) runs 100 GeV/amu and proton units at Fermilab and CERN are way bigger. The synchrotron has no real fundamental upper limit other that cost.
Of course, now if we start thinking the following question asks itself: if the ion acceleration energy is less than the 38.6 MeV we get from the decay chain, can we use this Earth-side to produce power? The critical observation is that we are spending kinetic energy to produce nuclear potential energy. The process is simply moving mass from two stable equilibria to an unstable equilibrium. The energy return rate could be enormously high if nature is kind (as if).
None of the processes involved has an inherent low efficiency. Particle accelerators use electromagnetic fields which transfer energy very efficiency. Radioisotope yields can be in excess of 90 %. The decay products are all charged particles, which can be efficiently captured or at least the system can produce high temperatures to drive a heat engine. Also realize that the kinetic energy of the ion is not lost when transmutation occurs. Instead it's converted to heat. That heat can be recovered and used to produce electricity, in effect reducing the power input for the accelerator. Check out this proposal for just that.
Unfortunately I have no idea how to estimate the energy needed for atomic fusion. It's probably significantly higher than 38.6 MeV or someone else would have already suggested the idea, right? As a potential power source for space applications it remains intriguing even if the energy balance is negative. There's also the possiblity that there's superior decay chains that I haven't noticed (Th-229 is good), especially those with higher Z than 240.
Zoom zoom...
5 comments:
You've been blogging up a storm. Very thoughtful, helpful posts. Thanks.
The easiest way I know to estimate the energy needed for fusion involving heavy nuclei is to look at the reverse process: alpha decay. An alpha particle flies around within a heavy nucleus, bound to the other nucleons by the strong nuclear force, but can, at times, find itself far enough from the herd that their electrostatic repulsion exceeds their strong nuclear attraction. The first time this happens, the alpha particle falls away to infinity. The lab-frame kinetic energy it so acquires, 1 to 2 MeV per nucleon, is just the energy an incoming particle needs for soft fusion. Maybe an eV or two less.
If a highly enriched 235-U core could burn completely it would yield 85 TJ/kg. Of course it can't, but the mentioned 16.3 TJ/kg sounds possible -- and you can turn it off.
--- Graham Cowan, former hydrogen fan
boron as energy carrier: real-car range, nuclear cachet
The number I often see bandied about for fission processes is 8.5 TJ/kg.
The real issue for novel isotope RTG versus a fission plant isn't energy content but power. While fission plants for mobile applications might run 500 W/kg, the Ra-228 can produce 20000 W/kg for quite some time. How much support infrastructure (power converters, radiators, etc.) you'd need for that radioactive material I'm not sure. There should still be a order of magnitude advantage for the RTG. Of course, as you noted, you can't turn a radiothermal battery off.
The basic problem is the lack of good naturally occuring isotopes for use as targets. Ra-226 appears to be the best target. You could bombard it with protons to make Ac-277 (which would be a good RTG material) and continue to form Th-228. Both are unstable and will decay, the Th-228 chain much faster.
The other option is to look at the Polonium isotopes but their decay chains are fast. Good for Earth power maybe but not for a space probe.
Robert,
I am looking for a power source for an MHD propulsion system that will use charged air for thrust. Which of the systems you refer to would produce the power requirments I suspect that I would need--to charge the air and then power up to 30 high temp super conducting magnet coils for the accelerator. If you do not have an answer, can you steer me in the right direction?
Thorium Power is on the Go:
www.thoriumpower.com/default2.asp?nav=technology_solutions
Post a Comment